Key message
Excessive production of reactive oxygen species (ROS) and oxidative stress play an important role in the pathogenesis of many eye diseases.
ROS stimulate cell death via apoptosis, participate in the activation of pro-inflammatory and pro-angiogenic pathways, and are associated with the autophagy process.
Introduction
Although higher eukaryotic aerobic organisms cannot exist without oxygen, the oxygen molecule is paradoxically dangerous to the very life forms for which it has become an essential component of energy production.1
In the ground state of oxygen, two unpaired electrons inhabit the oxygen molecule, and each belong to a different π* antibonding orbital. The electrons, which are rotated about their own axes in the same directions, have the same or parallel spins.2 In this state the oxygen molecule is most stable and therefore not very reactive. Activation of oxygen can be triggered by two mechanisms: either by absorbing enough energy to reverse the spin of one of the unpaired electrons forming a singlet oxygen3; or through monovalent reduction.4 The latter results in the production of reactive oxidative species which are by far more reactive than ground state oxygen.
Under physiological conditions, the antioxidant defense system within the body can easily neutralize or eliminate free radicals that are produced.5
If the production of reactive oxygen species (ROS) however exceeds the body’s capacity to cope with it, the resulting oxidative stress damages different macromolecules.6 Yet fortunately our body also has mechanisms either to repair damaged molecules such as the deoxyribonucleic acid (DNA) molecule or degrade damaged macromolecules such as proteins.7 If in addition to the age factor, this capacity is diminished the damage accumulates over time leading to detectable disease. Many diseases, as well as aging share the same mechanism, essentially based on oxidative stress. Activation of ROS leads to chronic inflammation when they are not effectively eliminated by protective mechanisms. The simultaneously appearing processes suggest that there may be a connection between both mechanisms.
Oxidative stress has been implicated in the pathogenesis of many ophthalmic diseases such as glaucoma, age related macular degeneration, cataract formation or diabetic retinopathy.8–10
In this manuscript we will focus on the role of oxidative stress in the pathogenesis of eye diseases.
The oxygen molecule (Ground state oxygen)
Oxygen in its electronic ground state is critically connected with life on Earth.
As soon as the oxygen molecule is electronically excited it becomes deleterious as it can form so-called “reactive oxygen species”.11 In its ground state (triplet state), the oxygen molecule is in its most stable state and only shows minimal reactivity. Oxygen is a diradical meaning that its highest molecular orbitals contain two unpaired electrons.12
But why is the oxygen molecule, a diradical in ground state, only minimally reactive? At ground state, the oxygen molecule has two unpaired electrons each located in its own π* antibonding orbital.13
These electrons rotate about their personal axis in the same direction which means that the two electrons have the equal spin and might consequently no longer be paired.
This is extremely rare in occurrence. Due to this particular “spin restriction” and the resulting stabilization of the triplet state by π-resonance stabilization of the two unpaired electrons, the oxygen molecule is only minimally reactive (Figure 1).14
Activation of oxygen
There are two different mechanisms by which the oxygen molecule can be activated.7 The first mechanism by which this can be achieved is by absorbing sufficient energy to reverse the spin of an unpaired electron.
Once the two electrons of the oxygen molecule in ground state have opposite spins, the oxygen molecule turns into a so-called singlet oxygen15 which is by far more reactive than ground state oxygen and reacts destructively with other molecules. Due to the opposite spins the two electrons in singlet oxygen can exist in an unpaired form as depicted in Figure 1, but also in a slightly less reactive paired variant.
The second mechanism of activation of ground state oxygen is by the step wise monovalent reduction of oxygen.16 Reduction refers to the gain of electrons or the loss of oxygen. As a result of monovalent oxygen reduction, superoxide anion radicals (O2.-) and hydrogen peroxide radicals (H2O2) are formed. Eventually, through further reduction, water is produced.
These reactive forms of the oxygen molecule are known as “reactive oxygen species”.17 Up to a certain degree, ROS are formed in our bodies as by-products of our normal physiological metabolism and they even have beneficial roles in cell signaling and homeostasis. However, they become harmful if produced in an extent which cannot be overcome by the body as explained later in more detail.
Damage to deoxyribonucleic acid (DNA) and proteins
Macromolecules such as DNA or proteins can be damaged by free radicals.17 DNA damage may affect normal cell replication and impact rates of apoptosis (programmed cell death) loss,18 or even the transformation of healthy cells to cancers. Fortunately, our body has diverse mechanisms to repair damaged DNA.19
Generally, DNA repair is achieved by two different mechanisms, reversing the chemical reaction that caused the damage or replacement of damaged bases.20
Damaged proteins may lose or change their function which in turn may compromise cellular function or even cause cell death.
Such damaged proteins are marked by the binding of ubiquitin to the amino group in the side chain of a lysine residue.21 The monoubiquinated protein is then linked with additional ubiquitins to form a multiubiquitin chain.22 Proteosomes identify these polyubiquinated proteins and degrade them.23
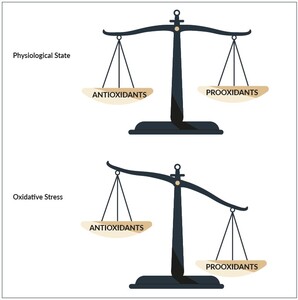
Oxidative stress
The healthy individual has been provided by various mechanisms to help neutralize ROS and cope with them.24 Under optimal conditions, the magnitude and rate of production of ROS is balanced out by its eliminations through the action of our bodies antioxidants.25 An antioxidant is any molecule that inhibits the oxidation of other molecules. ROS can therefore be dealt with by nature through a variety of mechanisms. If however, the production of ROS exceeds the bodies capacity to neutralize and cope with them the resulting oxidative stress (Figure 2) can damage different molecules as for example the DNA26 or protein molecules27 in our body. Fortunately, nature has also provided us with repair mechanisms. As explained above, these repair mechanisms help repair damaged DNA28 or eliminate the damaged proteins via proteasomes in our bodies.29 As long as the bodies repair mechanisms can cope with the amount of damaged molecules, no major structural damage occurs.
If, however the damage induced by oxidative stress exceeds the capacity of all repair mechanisms, the structural damage sums up and finally leads to a clinically relevant damage that we call disease.4,30
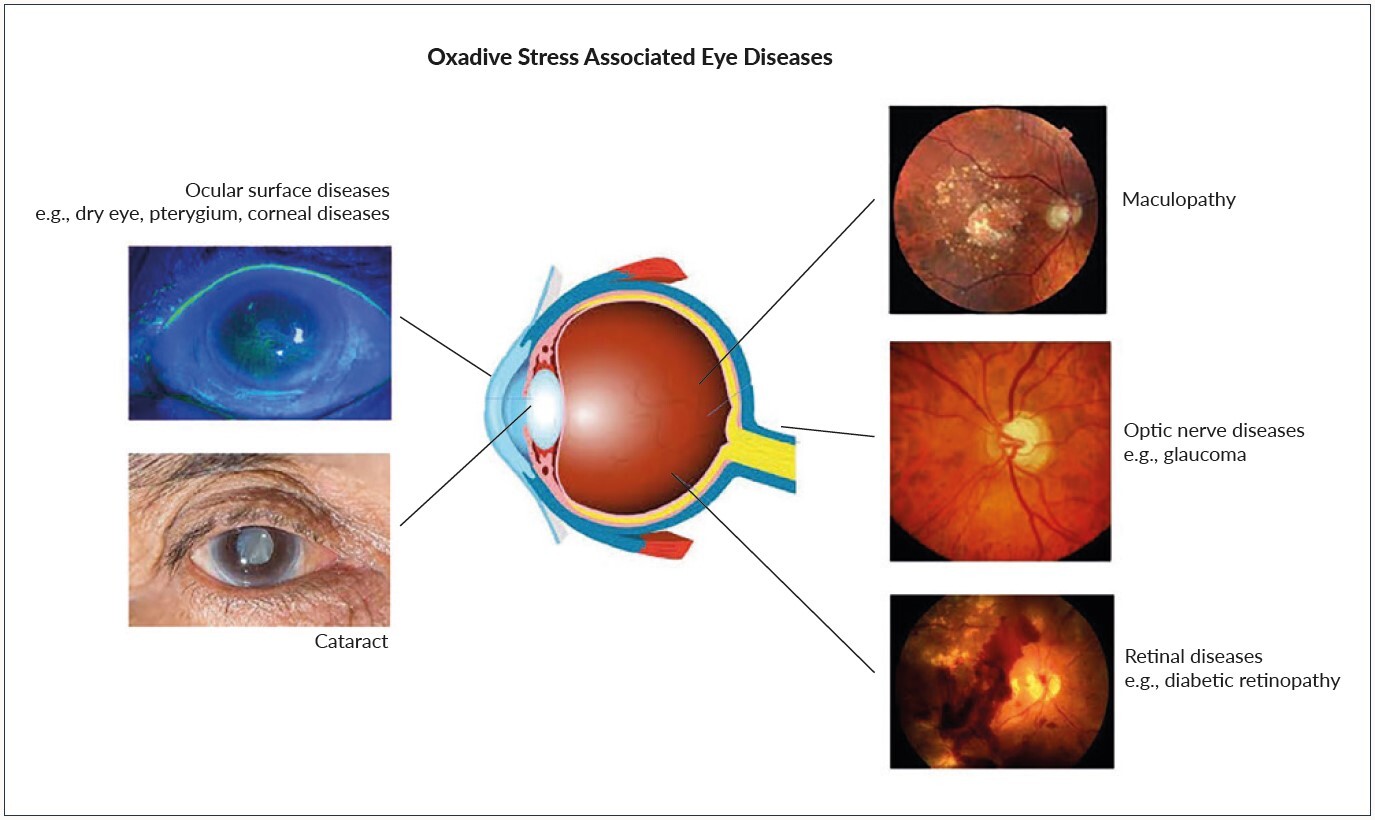
Due to the high exposure to external influences of our eyes, oxidative stress plays an important role in various ocular diseases.
Oxidative stress and eye disease
The eyes are unique as they are constantly exposed to sunlight, atmospheric oxygen, environmental chemicals and physical abrasion. In addition, these potentially deleterious ROS are generated internally by oxygen metabolism. Therefore the eyes are at risk to be exposed to higher levels of free radicals and to increased levels of oxidative stress.31
The accumulation of ROS in the ocular surface leads to dry eye disease and meibomian gland disease.32
Normal tear film is also rich in antioxidants like lactoferrin, uric acid and cysteine or ascorbate which are all acting protective against ROS.32 Therefore, tear film dysfunction can lead to infection and inflammation on the ocular surface and cause an increase in oxidative pressure, potentially leading to epidermal and glandular cell oxidative damage. This can result in a nonautoimmune dry eye syndrome.
The natural crystalline lens of the eye is a transparent avascular structure. Different metabolic activities, antioxidative mechanism and the sodium/potassium pump (Na+/K+ pump) facilitate keeping the lens clear.32 Numerous laboratory and epidemiological models suggest that oxidative stress is also a key factor in cataract formation which still represents a main causes of visual disability worldwide. In the cataractogenous process, ultraviolet (UV) rays in the ambient environment evoke ROS formation in the lens. Through this process, lens proteins modify sulfhydryl groups and become thiolated (reacted with, or converted into a thiol) or cross-linked by disulfide bonds. The result is a higher molecular weight of these proteins which through aggregation become insoluble and affect lens transparency. Polymerization and cross linkage of crystalline proteins precedes the morphological changes of cataract.33
Ideally, sustained high levels of reduced glutathione provide a protective effect, while depletion of glutathione causes damage to epithelial cells and fiber cells.
Furthermore there is a distinct relationship between corneal epithelial changes of the eye and corneal diseases such as keratitis, corneal oedema, photokeratitis and higher levels of oxidative stress.33
Oxidative stress plays an important role in diverse ocular diseases of the anterior and posterior segment.7
The main cause of irreversible blindness is glaucoma, a progressive, degenerative disease of the optic nerve.34 A dysfunctional antioxidant defense mechanism in glaucoma may contribute to the pathophysiology of glaucomatous neurodegenerative disease characterized by retinal ganglion cell death.35 Aqueous humor is known to contain ROS such as hydrogen peroxide and superoxide anion.
According to one hypothesis, chronic oxidative stress induced by these agents can compromise the function of the trabecular meshwork, the major pathway for aqueous outflow from the anterior chamber.
As trabecular meshwork is exposed to chronic oxidative stress throughout its lifetime, it has a sophisticated defense mechanism against ROS.
If a glaucomatous optic neuropathy occurs or progresses without an increased intraocular pressure (IOP), it’s called normal tension glaucoma. Ocular blood flow disturbances and resulting unstable oxygen supply have been suspected to be involved in this pathogenesis. A newer concept proposes that retinal vein occlusion based on venous constriction may be induced by vasoconstrictive molecules diffusing from neighboring diseased arteries and/or from other neighboring (hypoxic) tissues.36
Many patients with normal tension glaucoma have a basic constitution, which corresponds to the primary vascular dysregulation syndrome (Flammer syndrome). In this subgroup of patients with glaucoma, vascular dysregulation is present, and they generally present with a low body mass index, low blood pressure and often cold extremities.
Vascular dysregulation occurs when blood flow is not properly distributed to meet the demands of different tissues and could lead to over- or underperfusion. Vascular dysregulation can be primary or secondary and the result of unregulated local endothelial production of vasodilator and vasoconstrictor substances.26 The occurrence of vascular dysregulation often relates to increased levels of endothelin-1, leading to vasospasm.
Oxidative stress leads to the peroxidation of lipids and phospholipids thus triggering the onset of retinopathy.37 Diabetic retinopathy, one of the microvascular diabetes complications is classified as a progressive neurodegeneration. Diabetes is one of the most important health problems worldwide. Evidence suggests that oxidative stress plays an important role in the pathogenesis of this disease as ROS can directly oxidize proteins involved in the diabetic process. In conjunction with hyperglycemia, inducing endothelial cells damage, ROS are generated mainly in the mitochondria, thereby stimulating mitochondrial superoxide production.
There are four classical pathways known through which hyperglycemia can cause vascular damage: increased polyol pathway flux; increased intracellular formation of advanced glycation end-products (AGEs) and expression of the receptor for AGEs; activation of the protein kinase C (PKC) pathway; and activation of the hexosamine pathway. All these pathways are associated with the overproduction of ROS. Oxidative stress induced by epigenetic modifications can persist for a considerably longer time, even after hyperglycemia has ceased, this is called “metabolic memory”.
Age-related macular degeneration (AMD) is another leading cause of acquired blindness in developed countries. One of the main diagnostic features of AMD are the Drusen which consist of lipofuscin.38 Lipofuscin is produced during chemical reactions such as lipid peroxidation and it is deposited when the production of lipofuscin is beyond the disposal capacity of the photoreceptor pigment in the retinal pigment epithelium (RPE).38,39
RPE is particularly prone to ROS formation due to constant exposure to light, high oxygen consumption and high amount of polyunsaturated fatty acids.40
Drusen cause RPE degeneration and “geographic atrophy” of the fundus of the eye leading to severe visual loss.41 Oxidative stress is also involved in retinitis pigmentosa which is a group of inherited eye disorders, characterized by progressive rod photoreceptor apoptosis with consequent gradual death of cone cells, leading to blindness.42 Early symptoms of night blindness and peripheral visual field loss related to this disease may already occur in childhood or adolescence.43 Rods comprise about 95% of all photoreceptors while only 5% are made up by cones. Rods considered to be a highly metabolically active cells with an elevated oxygen consumption rate.44
When the disease retinal pigmentosa progresses the retinal oxygen consumption diminishes.45
Even though the oxygen demand is significantly decreased, the blood supply remains unchanged, leading to a hyper-oxidation state which causes oxidative damage. This oxidative damage influences all of the cells in the retina, including the cones and the rods which die by means apoptosis.46
Conclusions
Oxidative stress is an important risk factor in the pathogenesis of a variety of eye disease such as glaucoma, age related maculopathy or diabetic retinopathy (Figure 3).
In this review, we have demonstrated that by way of illustration in diabetes, hyperglycemia induces a series of metabolic abnormalities in the retina by producing reactive oxygen species (ROS) and subsequently initiating oxidative stress. This can in turn activate these abnormal metabolic pathways to produce more ROS, thereby creating a vicious cycle. Generally, oxidative stress causes retinal damage by inducing endothelial cell dysfunction, pericyte apoptosis and angiogenesis.
Inhibitors of these pathways may protect the retina from damage induced by high levels of glucose. As a summary, antioxidants are beneficial for pathogenesis of all ophthalmological diseases reviewed here as they reduce ROS production, neutralize free peroxynitrite or augment the antioxidant defense system.
Consequently, diagnostic tools enabling measurement of oxidative stress directly (comet assay analysis)26 or indirectly (retinal venous pressure measurement) are useful in a clinical setting. Moreover, patients can benefit by being informed on therapeutical approaches which could potentially reduce levels of oxidative stress through changes in lifestyle and antioxidative nutrition.
CONFLICTS OF INTEREST
The authors declare that there are no commercial or financial relationships that could be construed as potential conflicts of interest.
AUTHOR CONTRIBUTIONS
M. Bissell wrote the paper, M. Mozaffarieh revised the paper and made corrections, I. Fetian made a substantial contribution in proof reading and designing the illustrations.
FUNDING
This work received no specific grant from any funding agency in the public, commercial or non-profit sectors.
ACKNOWLEDGMENTS
Writing and editing assistance was provided by H+O communications Ltd., Zurich, Switzerland.
__the_last_two_electrons_of_the_oxygen_mo.jpeg)

__the_last_two_electrons_of_the_oxygen_mo.jpeg)